
“Knowing your forests from your trees: What’s falling afoul in the Woods? 1”

Acid rain | nitrogen saturation | isotopes | confirmation | soil fertility | trace elelment starvation | climate | N saturation | Solutions?
As all good foresters know trees need nitrogen no matter how much you give them forests just grow and ask for more. In fact 6 years ago. Ernst-Detler Schulze a plant ecologist at Bayreuth University in Germany made a big splash in the world forestry community by tying the woodland's appetite for nitrogen to northern Europe's acid-rain induced timber die-offs. Schulze argued compellingly that an acid rain of nitrates and other readily digestible forms of nitrogen stimulated trees to shunt unhealthy amounts of energy into growth. These trees. already suffering from pollution-induced chemical imbalances, in time became too weak to handle even the normal vicissitudes of daily life—insects, fungal blights,and extremes of weather (SN: J, 29 1989. p. a6).
Imagine Schulze's shock then, when he learned last year that much of the nitrate raining down on the upper forested slopes in Germany's Fichtelgebirge—a range of mountains within 2 miles of the Czech border—is not taken up by trees or nitrogen-hungry microbes in the soil. If atmospheric nitrate can pass through these forests without being touched by biology." he says "something is going wrong. Nitrate is passing through a soil with all sorts of organisms and none of them is interested in this nutrient any longer."
At a minimum, Schulze's new finding confounds scientists' understanding of how nitrogen cycles through woodlands. But even more provocatively it suggests that apparently healthy trees can in fact be on the verge of collapse. Many foresters read these data collected by Schulze and his colleagues from heavily polluted stands of Norway spruce as signaling that these woodlands can become saturated with nitrogen in a self-accelerating process that poses a threat to forest vitality worldwide. Some also suspect the timber will adapt to nitrogen saturation—by growing more slowly. This would force the wood-products industry to wait longer for such trees to reach harvest. This is troubling to the environmental community because these forests are defective at mopping up the carbon dioxide releases that threaten to trigger a global warming.
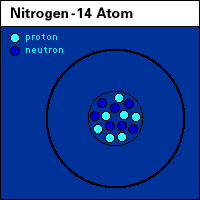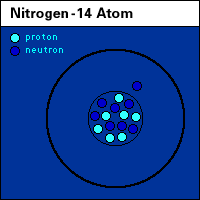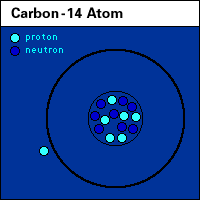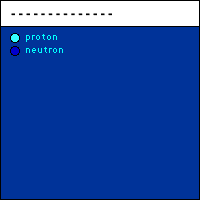 One neutron spells the difference between two naturally occurring forms (or isotopes) of nitrogen: nitrogen-14 and nitrogen-15. Similarly a pair of neutrons distinguishes oxygen 18 from its lighter sibling, oxygen-16. For reasons not now fully understood, the nitrate (NO3) in combustion-generated air pollution has more nitrogen-15 and oxygen-18 than does the nitrate produced by soil bacteria.
One neutron spells the difference between two naturally occurring forms (or isotopes) of nitrogen: nitrogen-14 and nitrogen-15. Similarly a pair of neutrons distinguishes oxygen 18 from its lighter sibling, oxygen-16. For reasons not now fully understood, the nitrate (NO3) in combustion-generated air pollution has more nitrogen-15 and oxygen-18 than does the nitrate produced by soil bacteria.
Several research groups have observed nitrate in water draining out of forest soils recently. In theory there should be little, if any present. To investigate this curiosity Schulze and his coworkers employed a novel technique: They determined the ratios of those heavy and light isotopes of nitrogen and oxygen in spring-fed streams. Because heavy nitrate comes only from air pollution and light nitrate only from soil microbes. Schulze explains "they could now trace that nitrate leaving forests to distinct sources,'' either, soil bacteria or air pollution.
The Germans found that 16 to 90 percent of the nitrate leaving two healthy-looking Fichtelgebirge stands bore air pollution's signature they reported in Dec. 2, 1994. In three “slightly declining" stands of spruce 23 to 30 percent of the nitrate in runoff came from air pollution. But the isotope tracers showed that fully 60 to 100 per cent of the nitrate leaving two dying stands of trees came directly from the atmosphere.
Other studies clearly says that the phenomenon the authors describe is correct . . . [that] “nitrate [here] tends to pass through soils rapidly" says John Aber at the University of New Hampshire's Complex Systems Research Center in Durham. However he adds “this is exactly what microbiologists would predict.” It's harder for soil microbes to use nitrate than the more readily digestible ammonium (NH4) another abundant nitrogenous pollutant. So while a surfeit of nitrogen is available—or the biological activity of the forest has been slowed by acidification — timberlands may slake their thirst for nitrogen without using much if any nitrate.
But to James Holenbeck a research forester with the U.S. Forest Service's experiment station in Durham N.H., the idea that atmospheric deposition could pass through forests without cycling through trees or microbes is very surprising— and presents some interesting concerns." [ Trees need soil microbes to convert nitrite to nitrate (10 percent of soil nitrate remains in a seriously damaged stand of fir (Abies) forests.) ]
Chief among them is “the long-term effect this would have on soil fertility." notes forest ecologist David Van Lear of Clemson University. It's a matter of chemistry. Being negatively charged, nitrate cannot move through the soil without dragging along cations (positively charged minerals). The first cations to leave the soil will be the essential nutrients calcium and magnesium. "So the more atmospheric nitrate that's moving through, the more calcium and magnesium that will be leached from the soil," Van Lear says.
The result, explains Walter C. Shortle, also with the Forest Service in Durham, is that over a matter of just decades, a forest can be transformed from a system starved for nitrogen to one sated with nitrogen but starved of these essential alkaline (base) cations. And if you develop an insufficiency of these base cations at the same time you've got plenty of carbon dioxide and nitrogen, young trees will adapt." he says. "They'll look perfectly happy -- like little bonsai trees." -- While stunted and slow-growing, he says, for all practical purposes “they would be healthy.'' Forest hydrologist Gregory Lawrence of the U.S. Geological Survey in Albany, agrees that if a forest is not utilizing nitrogen effectively, this suggests productivity is dropping." And if productivity drops, he notes, “you're not going to get as much wood."

Those who attempt to model how well future forests can store carbon — and stave off a global warming — “may receive a rude awakening if they don't begin accounting for acid rain's ability to radically alter the nutritional needs of timberlands,” Shortle, believes. Indeed, he sees this as one of the primary take-home messages in the German data.
Lars O. Hedin of Cornell University sees another. The environmentally unsettling implication of these [new German] results is that forest decline might be a self-accelerating process," he says. Specifically, he refers to the fact that if nitrate saturation fosters a leaching of cations, it will also foster growing acidity of the soil. Acidity damages plant roots, may inactivate microbes that would use and store nitrogen, and encourages the movement of aluminum—which is toxic to plants (and to aquatic life if it leaches into streams).
So as nitrogen saturation occurs. the ability of a forest to buffer acid rain progressively diminishes. The result, Hedin worries, might prove a spiraling decline that only accelerates with time. Lawrence concurs that "there really does seem to be the potential for this spiraling effect that continues until the whole system crashes" And he credits Schulze's team as the first to recognize nitrate in spring water, N-15, an early warning that such a forest had reached its nitrogen saturation point.
Admittedly, Hedin admits, nitrogen deposition is not as high in the northeastern United States as it is in Germany: However, he suspects the problems Schulze's team is observing may point to what's in store for others. Indeed. Hedin asserts, unless pollution trends in the United States and elsewhere change, “you could argue that the difference between them and Fichtelgebirge is just a matter of time "
If atmospheric nitrate-15 can pass unchanged through even our healthy stands where no [tree] damage is visible,'' Schulze's team observes, “This means we have a process going on in these soils which will mean even more severe damage in the visible future"— probably the next several decades. To Hedin and others, these apparently healthy stands represent sick trees at high risk of decline or even sudden death—the arboreal counterpart of a person who goes to the doctor with undiagnosed high blood pressure and an elevated cholesterol concentration. Can't foresters doctor their woodland patients by liming them with calcium and perhaps magnesium to reverse the acidification process and their developing mineral imbalances?
Yes, but such treatment falls short of a cure. The new data hint that two of the slightly declining sites that Schulze's group analyzed have been limed. And they still allowed 93 and 30 percent, respectively, of the atmospheric nitrate to cycle through and out of the soil unaltered. “Clearly,” Schulze says, “as soon as the soil is limed, the microorganisms get a kick out of it." And not just those that could use and lock up nitrate from the atmosphere into trees and other long lasting stores of nitrogen. He notes that some invigorated microbes also "start to chew up lignin and other carbon structures in soil, which we would ordinarily describe as permanent humus. The end result: Instead of allowing forests to store more carbon, liming may actually facilitate the milling of carbon by soil microbes. And some of these newly invigorated microbes will also convert ammonium into nitrate. This nitrite, if formed nearer the forests soil surface, will aggravate the leaching of cation nutrients from the root zone, further increasing the forest's risk.
So what's the solution? Most foresters believe that polluters must cut back on emissions that rain out as nitrates, ammonia (NH3), and ammonium (NH4). But that may not prove as effective as once thought, Lawrence argues. Just a few years ago, he recalls, pollution analysts working on revisions to the Clean Air Act argued "that you could just stop emissions for a little while and things would recover in 10 years or so." That now appears unlikely, he says.
Cutting emissions would slow a forest's rate of degradation, he acknowledges, "but not rebuild the cations—like calcium—back up in the forest floor." Indeed, he notes, acid leaching in some places appears to have removed in just 40 to 50 years some 500 years' worth of calcium laid down naturally.
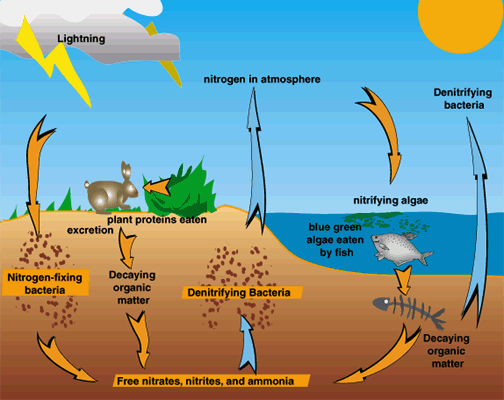
That's why Schulze urges another strategy for attacking the problem: cycling nitrate from acid rain through denitrifying bacteria. These organisms, which live in oxygen free conditions such as bogs, break nitrate down. "If we would not plant our forests too close to springs — but instead allowed that land to remain boggy—we might- mop up much of our nitrate," Schulze argues. But this “would only clean the water of surface springs"—it would not protect tree roots against acidification. However, he says, it does offer yet another argument for preserving existing forests, restoring some, and replanting new ones.
Water draining through [an] old growth such as that of temperate evergreens in southern Chile contains the lowest concentration of nitrate ever reported (100 to 200 parts per trillion). As such this forest— undisturbed by human activities— represents an ecological baseline against which others can be compared.
"The nitrogen cycle in unpolluted southern Chilean old-growth (left panel) forests compared to North American temperate forests (right panel), which are subjected to higher nitrogen deposition derived from anthropogenic sources. Arrow widths indicate the relative magnitude of nutrient fluxes.
N inorg Inorganic nitrogen, N org organic nitrogen.
Organic forms (dissolved and particulate) are more important in the outputs from forested watersheds in Chile than in North America. Inputs and outputs in most North American forest are dominated by inorganic nitrogen. Inputs in Chilean forests are also dominated by organic over inorganic forms of nitrogen. Notice that nitrogen fixation increases in relative importance in southern forests relative to atmospheric inputs."
Springer-Verlag, "Images"
cations these are positively charged ions, the opposite of anions or negatively charged ions.
acid rain | nitrogen saturation | isotopes | confirmation | soil fertility | trace elelment starvation | climate | N saturation | Solutions?
§§§
1) What is the precise character of the scientific problem this forest study examines?
2) Identify the disrupted cycles that forests depend on from this article?
3) What are the costs of the disruption to the forest (ecologically)?
4) What has been the impact of the remedies used to mitigate or correct these threats?
5) How are those ecological costs translated into economic losses?
6) What clues do denitrifying bacteria provide us with about this problem?
![]()
“Knowing a forest from the trees" is a phrase to convey a common problem of knowing so may details that one can lose sight f the context. This is too often so that many people get lost in the details because they re overwhelmed. The metaphorical phrase for this condition of detail overload is that you "can't see the forest through the trees."
"What’s falling afoul in the Woods?" to "fall afoul" means "to encounter conflict or difficulty with something." In this case the problem of acid rain leaching nutrients from the soil that sustain forests downwind from coal-burning plants.
![]()