- RESEARCH
- TEACHING
- PUBLICATIONS
- LINKS
- PERSONAL
Research Interests
Mission Statement
Over my first couple of years at Rollins, it is my strong desire to put together a physiological psychology laboratory that provides an opportunity for Rollins students to get experience with classic psychobiological techniques. We are already accruing equipment that can be applied to animal psychophysical experiments, and will soon marry that procedure with the ability to make precise stereotaxic lesions and then perform histological and microscopic analyses to verify the location of the lesions. In the near future we will have the ability to explore neural coding issues with in vivo electrophysiological techniques. Finally, we hope to augment physiological and behavioral studies with neuroanatomical investigations. Guiding the development of the laboratory will always be the overarching goal to involve Rollins students in the research process.
Goal of the Research
One of the most fascinating questions on the border of psychology, neuroscience, and philosophy is the question of how physical tissue (the nervous system) can create psychological mental states. While we fool ourselves into thinking that we respond to objects and stimuli "out there" in the real world, it can be easily shown (through sensory illusions, for example) that our behavior is ultimately controlled by what's "in there", that is, the psychological world that our brains create.
We are perhaps a long way from understanding how this is done (but perhaps a lot closer than we used to be). A first approach is to understand how our brains respond to incoming information, that is, how do our brains represent the sensory stimuli constantly assailing our visual, auditory, somatosensory, olfactory, and gustatory receptors? The first approach to figuring out how our brains create the intangible impression of "sweetness" is finding out what neural activity is correlated with the interaction of our tongues with sugar, and how this representation differs from that evoked by salt, or citic acid, or, for that matter, the color red.
That requires looking "in there". Noninvasive techniques like functional magnetic resonance imaging do not have the kind of spatial and temporal resolution sufficient for answering questions of neural coding. The invasive techniques required, however, are ethically impossible in humans in most situations. Thus I have chosen to use several common rodent models to answer psychobiological questions about perception. These include the albino rat as well as several strains of mice. Recently, in collaboration with investigators at the University of Tennessee Health Science Center, we have begun using congenic lines of mice with phenotypic properties of interest. One such line has over 99% of the DNA of a strain that cannot taste the aversive substance sucrose octaacetate (SOA), but contains a stretch of DNA around a single gene that apparently gives them the ability to taste this substance.
Once you leave the realm of humans, though, you run into another problem: how do we know what a human finds "sweet" is the same things that a rat or mouse finds sweet? How can we tell if they can taste anything at all? Using sophisticated operant paradigms and customized apparatuses (see figure), we employ a battery of psychophysical techniques that give us a window into the sensory worlds of these animals. Only after that is done can we try to relate that to what is going on electrophysiologically or anatomically in the brain.
Active Projects
Salt Taste
For a long time it has been known that salt is transduced by two separate pathways, one "amiloride-sensitive" and one "amiloride-insensitive" based on the antagonistic action of the molecule amiloride. Several suggestions for the mechanism of the amiloride-insensitive pathway have been proposed; recent interest has focused on cetylpyridinium chloride (CPC), a constituent of mouthwashes and fluoride rinses. While attempting to study its antagonistic effects on salt taste, our PSY 333 class made the observation that CPC itself is aversive. We are currently studying CPC's aversive properties, as well as trying to find a way to behaviorally analyze its potential effects on salt transduction without the analysis being confounded by its aversiveness. Recently, CPC has been shown to act at a VR1 channel, so we are also testing the effect of more specific VR1 antagonists on salt taste.
Strain Differences in Taste
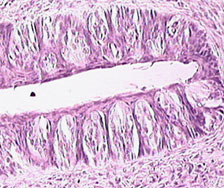
In collaboration with John Boughter at the University of Tennessee Health Science Center, we are currently looking at strain differences in bitter taste in mice, as well as differences in the taste of basic compounds like Ca(OH)2. Our original work focused on C3 and the SWR mice, which differ in their sensitivity to SOA, an chemical that is bitter to humans and aversive to the SWR mice. SOA avoidance appears to be controlled by a single gene, SOA, which is strongly implicated as a receptor molecule. Our research is enhanced by studying a congenic mouse strain (see figure), which is genetically identical to the insensitive strain (C3) except for less than 1% of its genome containing the SOA gene from the SWR strain. If SOA codes for a receptor, how is that receptor distributed in the taste buds of the mouse? We are examining this question using nerve transection studies.
An even more intriguing strain difference seem to exist for basic compounds. Although both SWR and C3 mice appear to taste these compounds, the SWR mice find the taste aversive whereas the C3 mice find it pleasant. To our knowledge, this is the first example of a strain difference where the mice differ not only in the intensity of a taste, but in its hedonic value. Is this difference peripheral or central? Is it controlled by one or a few genes? Do the mice differ in the perceived quality of the basic compounds, or only in its hedonic value?
Fatty Acid Taste
For a long time, it was thought that fats are sensed by the olfactory and somatosensory systems, but not by the taste system. Evidence has begun to accrue, most compellingly from my collaborator Dr. David Pittman at Wofford College, that fats may also be "tasted". If this is true in humans as well, then, for example, the success of fat substitutes in the diet will depend critically on understanding the gustatory sense of fats in addition to their other sensory properties. We have begun to collaborate with Dr. Pittman on the contribution of taste to the ingestion of fats.
Control of Licking
John Boughter has recently identified an interesting strain difference in the rate at which mice (the B6 and D2 strains) lick from a drinking spout. Licking, like many motor behaviors, is governed by central pattern generators, which are circuits of neurons in the brainstem and spinal cord which coordinate rhythmic, carefully timed movements. The identification of a strain difference in B6 and D2 mice could be especially useful in determining genetic contributions to these central pattern generators. Experiments are underway to establish whether the strain difference has a neural basis (or is due to less interesting factors like muscle constituency or size of the animal).
Other Questions
We have also become interested in what I call "the quinine paradox". Rats (and people) can detect quinine at extermely low concentrations (i.e. at concentrations about 1-10,000 times lower than the lowest concentration of sugar that can be detected), but electrophysiological responses are difficult to record at these low concentrations. In other words, rats have a strong behavioral response (avoidance) to concentrations of quinine that don't seem to be registered in the brain. We are examining this problem behaviorally and electrophysiologically.
Other questions which are being pursued: What is the role of saliva in taste? What is the capacity of the taste system to regenerate after damage? Does the brain have the ability to change in response to gustatory experience as is the case with the more encephalized senses of vision, audition, and somatosensation? What is the role of taste in the control of ingestive behavior?
The answers to many of these questions are already being explored with the help of several Rollins students, and, we hope, will inspire many more questions in the future.