 T
T The relation of Matter and Energy is complex.
The relation of Matter and Energy is complex. 

Gell-Mann, The Quark and the Jaguar,
"All matter possesses energy and all energy is associated with matter."
"When people refer carelessly to matter being converted into energy (or vice versa), they mean simply that certain kinds of matter and energy are being converted into other kinds. For example, an electron and a related (but oppositely charged) particle called a positron can come together and turn into two photons, a process often described as annihilation or even as ‘annihilation of matter to give energy.’ However, it is merely the transformation of matter into other matter, of certain forms of energy into other forms.”
p.
124.
“Just as the electromagnetic force between electrons is generated by the virtual exchange of photons,”
p.182
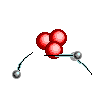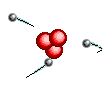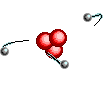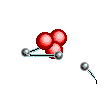
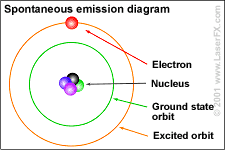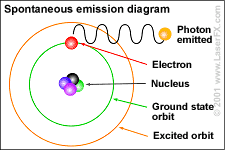
Atoms are
like clouds of electrical charge encircled by electrons  traveling at the speed of light about the dense nucleus (pictured above as red).
Two basic forces of material existence emerge from atomic nuclei, the strong
or binding force and the weak or electro weak force that accounts for radiation.
traveling at the speed of light about the dense nucleus (pictured above as red).
Two basic forces of material existence emerge from atomic nuclei, the strong
or binding force and the weak or electro weak force that accounts for radiation.
Electrons carry the electromagnetic (now argued to be the"electro-weak") force, as a flow of electrons is an electrical current.
All electrons have:
| symbol | particle name | mass | charge | spin |
|
e
- |
electron |
1
|
negative | 1/2 |
|
e |
electron neutrino |
<1
|
uncharged | 1/2 |
|
e
+ |
positron |
1
|
positive |
Physicist Brian Greene calls the photon "a 'bundle' of light" and more specifically a carrier of force from the fifth dimension through our world or as he says "the messenger particle of the electromagnetic force."
Fabric of the Cosmos, p. 539.
"Since neither the neutron or the proton is elementary,
however,
such reactions are not the basic processes, which involve quarks instead:
1. An electron turns into an electron neutrino while a u quark turns into a d quark.
2. An electron neutrino turns into and electron while a d quark turns into a u quark.”Are strings more basic than quarks?
pp. 188-189.
“How can there be so many elementary particles?”
p. 195.
In addition we have the photon, and three intermediate bosons of the weak interaction.
The higgson completes our list of elementary particles demanded by the standard model.
"Let us add up the total number. We have eighteen quarks, three electron-like particles, and three neutrinos, making twenty-four fermions in all. With their antiparticles, we have forty eight. Then there are the known quanta: the eight gluons,the photon, and three intermediate bosons for the weak interaction., brining the total to sixty. With the higgson, we have sixty one.”
p. 195.
"The universe was transformed beyond recognition during the course of the twentieth century. At the end of the nineteenth century space and time were generally understood to be absolute categories, unchangeable and unvarying in their properties regardless of the position and speed of the observer."
Bowler & Morus, Making of Modern Science, p. 296.
"The theories of special and general relativity required a wholesale rethinking of o of the relationship between space and time. Quantum mechanics called for a systematic reconsideration of the relationship between cause and effect, as well as a reassessment of just what it might be possible to know about the fundamental structure of matter.
Making of Modern Science, p. 253.
The photon then, represents –in its fullest importance–
a revolution in understanding the heavens, the earth, and the mini-cosmos we
share because photons and electrons exist within everything.
"Photon" is the name given to particles of light in the quantum mechanical understanding.
In interaction where the classical and quantum mechanical understandings of light agree they are fully equivalent to electromagnetic waves.
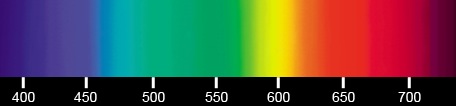
| The photon's wavelengths in the visible spectrum. | |
|
 |
|
Light spectrum
Strings
|
A technique of discovery | Photographs, impact of

atoms
Bohr's idea
complexity
doubt
Energy formula
photons
radiation as energy
See this terrific science web site
![]()
Atoms | Bohr | Electromagnetism | Light quanta | Quantum biology | Quantum qualities | Quantum Universe | Radiation is