 "Mad
as a hatter!"
"Mad
as a hatter!"Navigating the site:
Mercury is a liquid metal that reflects how ecological accounting, externalities, and waste informs design.
"Mercury is a heavy metal that is liquid at room temperature." It was, because of its shining viscosity, that mercury was referred to as quicksilver in the ancient world.
Ehrlich, Ehrlich, & Holdren, 1976, p. 57.
Mercury is vaporized (into the air) at relatively low temperatures forming a "colorless, odorless and tasteless" exhaust gas.
Zumdahl and Zumdahl, p. 1020.
Many of the applications where it is used suggest that "mercury is recyclable in principle."
"By how much have the activities of civilization altered the natural flows of mercury through the environment?"
Ehrlich, Ehrlich, & Holdren, 1976, p. 571-72.
Varieties | Trace amounts? | the threat | further considerations | uncertainty | scale | sources
Trace elements are materials that are small in matter but have great importance on wide scales.
"The mercury content of coal varies widely, ...ranging from .01 PPM to 33 PPM by weight."
p. 571.
In studies of one coal fired electrical generating plant, "It was shown...that more than 90 percent of the mercury went up the stack and less than 10 percent remains in the bottom ash."
"The mercury content of petroleum is even less well measured....it was found to be 10 PPM in one (probably exceptional) circumstance."
p. 572.
Mercury content of the Greenland ice sheets has been measured to reveal the prehuman accumulations in the atmosphere as deposited by rain and snow in Pleistocene times and earlier epochs.
1000 metric tons per year was once considered the "natural background" or ambient level of mercury prior to industrial civilization.
From 1950-1970, for example, "a doubling in the mercury content of precipitation," was "suggested by ice samples from the Greenland ice sheet."
p. 572.
Varieties of mercury:
Inorganic mercury: mercuric sulfide, (HgS ---> HgS04)
Organic mercury: phenyl, methyl, methyloxyethyl mercury. methyl (CH3Hg+)
"It was discovered in 1969 that microorganisms widespread in sediments are capable of forming methyl mercury and dimethyl mercury from inorganic mercury compounds"
The capacity of soil organisms to "fix" inorganic mercury vapor or salts into soluble, or organic mercury available to living creatures "has made mercury poisoning from high levels of methyl mercury in fish so serious a threat."
In addition to industrial chemical processes (caustic soda making) mercury is a by product -- quite literally-- an externality of the electricity generating process.
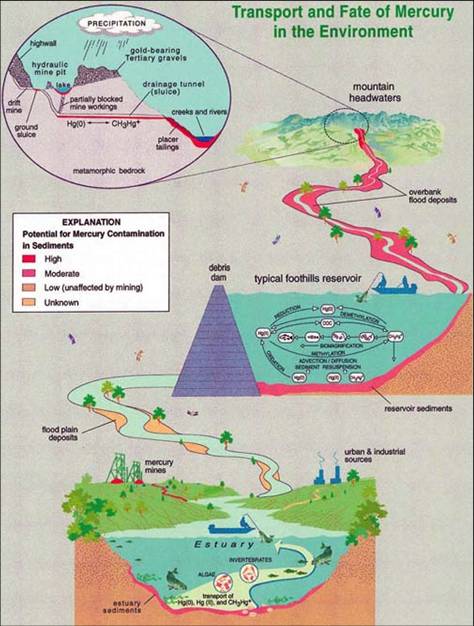
The use of oil and coal adds as much as two to four times the mass of mercury to the ambient or existing natural background load of inorganic mercury. Soil, mud or submerged organisms, by converting inorganic into organic mercury make some portion of this increased load available to consumers of plankton, algae, or other plants and bacteria.
Biotic considerations include: (or biological magnification) "pollutants may be...stored in the bodies of an organism and passed on up the food chain in a process called biological amplification.
The chemical is stored in the body of the predator, where the successive doses accumulate and become more concentrated....Thus, the concentration of the toxin amplified at each link in the food chain. Because heavy metals are toxic to many organisms at even small concentrations...."
Mark Bush, p. 278.
Varieties | Trace amounts? | the threat | further considerations | uncertainty | scale | sources
Designed to fail?
"Ecological design is not bound to a particular scale. It provides a way of uniting diverse design perspectives --and the different scales they represent-- and testing them against strong ecological constraints."
"New glazing technologies, appropriate choices of building materials, and sensible solar siting can together eliminate the need for a conventional heating system."
Sim Van der Ryn, 1996, page 44.
"determine the characteristic scale of acid rain.
"Isn't the true scale the of acid rain molecular, embedded in the intricate process chemistry of coal combustion?
Acid rain involves the flow of various contaminants across many levels of scale."
page 34.
We have used design cleverly in the service of narrowly defined human interests but have neglected its relationship with our fellow creatures. Such myopic design cannot fail to degrade the living world, and by extension our own health."
Page 9
"Ecological design,...provides specific ways of minimizing energy and materials use, reducing pollution, preserving habitat, and fostering community, health, and beauty."
Page x.
A designer is anyone "involved in shaping the physical details of our daily experience." p. 8.
"Dumb design is wasteful of energy and resources. It is polluting, extravagant, and profoundly dangerous. Unfortunately we are surrounded by it." p. 10.
Varieties | Trace amounts? | the threat | further considerations | uncertainty | scale | sources
Uncertainties:
Just how much mercury exposure is harmful, over what duration and in what form?
US standard for mercury exposure was first based on experience with the felt for hats and later electronics industries.
Air concentration: comparing US and Russian minimal tolerances, respectively.
100.0 micrograms of inorganic mercury per cubic meter / 8 hour period
.01 micrograms of inorganic mercury per cubic meter / 24 hour period
Ehrlich, Ehrlich, Holdren, 1977, p. 573.
Matrix based on a Spectrum of risk:
|
most
|
some
|
least
|
unknown
|
|
mercury vapor
|
methyl mercury
|
mercury amalgam
|
global budget
|
| all people | mining | vapor gas | |
| adults | pregnancy | caustic soda | outgassing |
| nursing mother | chlorine | leaching | |
| children | children | tooth fillings | bottom ash |
"A tentative standard for mercury in drinking water has been set (in 1970s) at 5 ppb." *
Uncertainty persists with regard to the mercury pollution problem because it is a natural component carried along by living systems and is recycled from inorganic to organic forms by bacterial organisms and made available to consumer creatures.
Mercury vapor is the most serious threat; when inhaled it passes the alveoli - blood barrier in the lungs and elemental mercury enters the bloodstream. Once in the bloodstream "the mercury (HgO) cation can denature proteins (break them up) inhibit enzyme activity and disrupt cell membranes." Once this occurs, "Death often results from kidney or respiratory failure."
( Zumdahl p. 1020.)
Measurement:
1 microgram per cubic meter = 1 part per billion (ppb) by weight.
Ehrlich, Ehrlich, Holdren, 1977, p. 573.
MercuryChemistry, Zumdahl and Zumdahl, (Boston: Houghton Mifflin, 2003). p. 1020.
Ecology of a Changing Planet, Mark B. Bush. (NJ: Prentice Hall, 2000), pp. 279-285.
Ecoscience: Population, Resources and Environment. Paul R. Ehrlich, Anne H. Ehrlich, John P. Holdren. ( SF, Ca.: W.H. Freeman, 1977). pp. 567-573.
Ecological Design, Sim Van der Ryn & Stuart Cowen. (Covelo, Ca.: Island Press, 1996). pp. x-45.
G. Tyler Miller, Environmental Science.
Varieties | Trace amounts? | the threat | further considerations | uncertainty | scale | start
Science Index | Site Analysis | Population Index | Global Warming Index | Nature Index