Waves and radiation
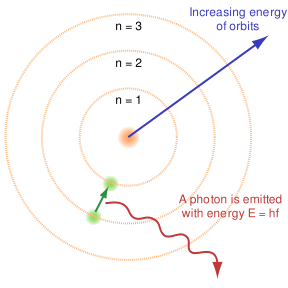
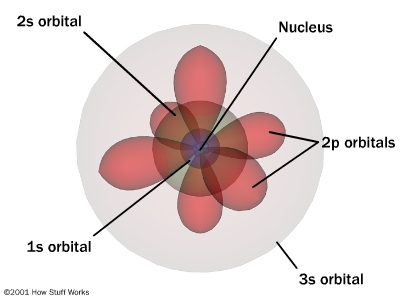
The US EPA describes the world of energy in which we dwell as filled with "Radiation that has enough energy to move atoms in a molecule around or cause them to vibrate, but not enough to remove electrons, is referred to as "non-ionizing radiation." Radiation that falls within the ionizing radiation" range has enough energy to remove tightly bound electrons from atoms, thus creating ions." This is the basic condition in which we all dwell the world of "waves and radiation," because light can be understood as waves with extraordinary frequencies. But simultaneously, light has been defined as particles, or packets of energy called quanta.


Animation of the above concept.
The periodic table of elements and their relation to the environment.
Electromagnetic charge and wave frequency displayed as a dialectical spectrum
Radiation arises from the atomic level as both the strong and weak forces.
really quarks & are
restless, often powerful sources of energy.
Members of the Quantum universe!