This site:
Atom models
![]()
![]()
![]()
![]()
![]()
![]()
The nuclei, is the plural of the word nucleus meaning a center or central mass of atoms.
"through the gateway of the atom . . . in a world which our senses cannot experience. There is a new architecture here, a way that things are put together which we cannot know: we only try to picture it by analogy, a new act of imagination."
Jacob Bronowski, The Ascent of Man.
This may be a confusing term because nucleus refers to the center of a cell, and the core of an even far smaller unit of material existence: atomic nuclei.
By definition there is no one nucleus. There are atomic nuclei just as there are cell nuclei for every eukaryotic cell but there are far more atoms than cells since cells are comprised of numerous atoms.
Since there are multiple cells which are themselves comprised of even more multitudinous atoms, the abstraction of a nucleus is handy when we try to label the parts of either 1) the organic cell or 2) the inorganic atomic elements.
Atoms | nuclei | protons and mass | electrons and light | neutrons and time | curve of binding energy
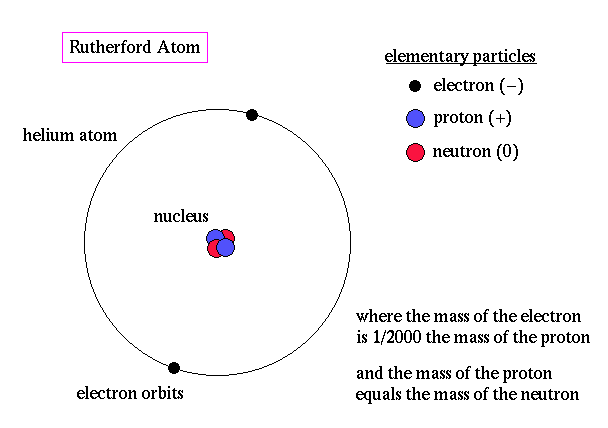
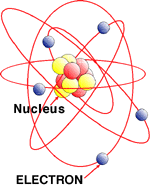
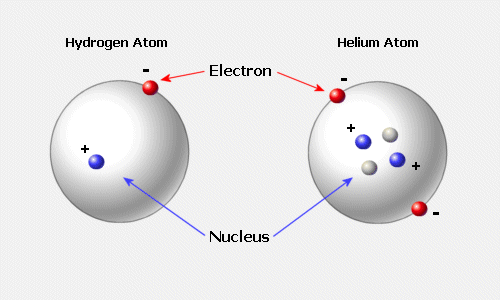
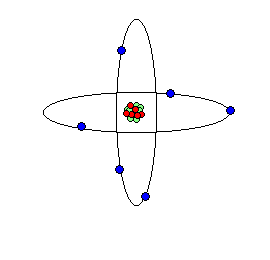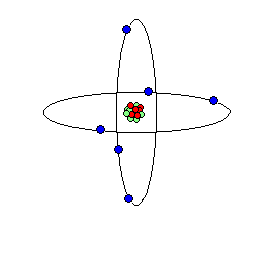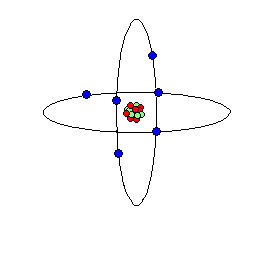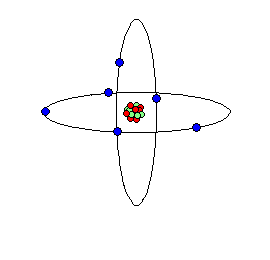
All atoms are massive nuclei surrounded by incredibly fast moving centers of negative charge called electrons:
 Electrons are associated with atoms.
Electrons are associated with atoms.
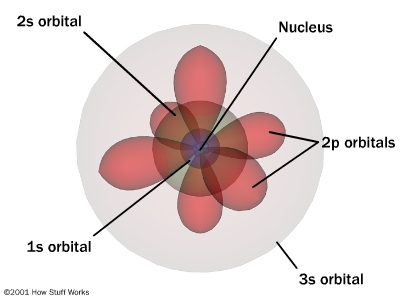
The nuclei of atoms are encircled by electron orbitals like clouds since the electrons move at light speeds approaching 186,000 miles per second. At that speed and in this confined space it is logical to ask: where are the electrons? Everywhere!
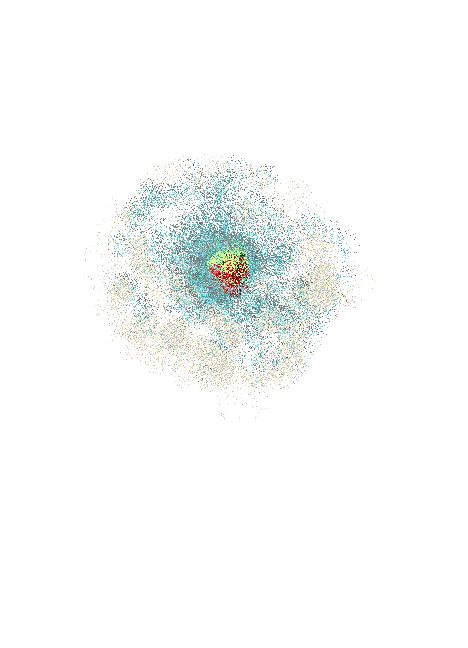 Contrary to impressions atoms are mostly empty space!
Contrary to impressions atoms are mostly empty space!
Clouds of charged particles, that repel other charged particles give us the impression that matter is hard, dense and impenetrable.
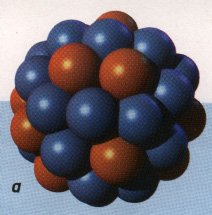
In the nuclei of Atoms there are protons and neutrons shielded by an electrically charged wall or shell of electrons. In the artists rendering below neutrons are blue and protons are red.
Atomic nuclei
proton is a positively charged subatomic particle

and a
neutron is an uncharged subatomic particle.
unbound neutrons decay into protons
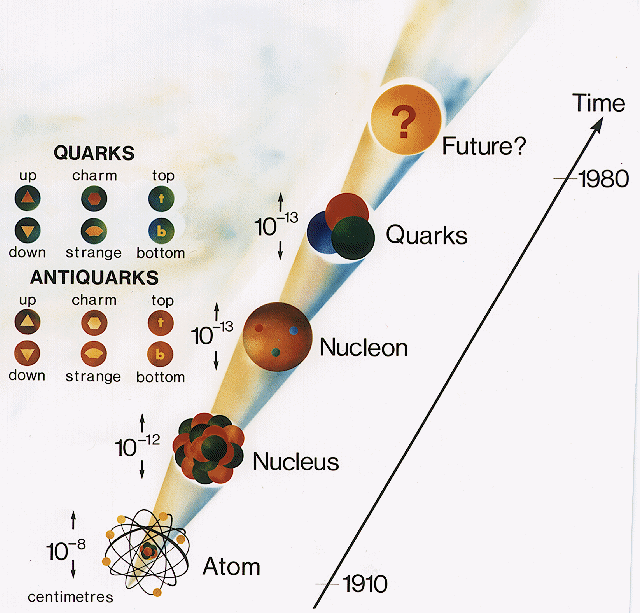
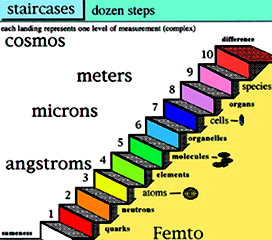
In four distinct layers or more, sub atomic particles are themselves comprised of even smaller units.
The layers of the hidden dimensions names
Strings
Nuclear Atomic Representations of the atom from our "billiard ball" metaphor may distort our imaginations' attempts to visualize these unseen atoms and their subatomic parts.
Extremely small and fast moving --electrons can move among atoms– creating electrical fields.
Two views or conceptions of what a atoms are and how they are constructed from sub-atomic particles:
A second look reveals:
Atoms harbor worlds within worlds.

H is for hydrogen, He is for helium, C is for carbon, all are essential atoms for life on Earth.
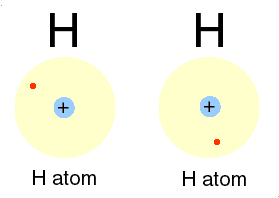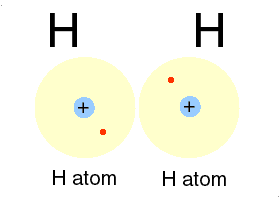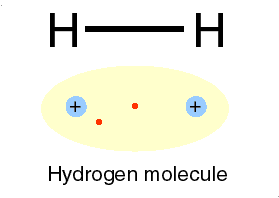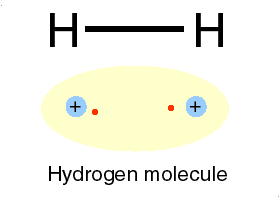
Hydrogen is H
Isotopes of H
Deuterium & Tritium
Two isotopes of Hydrogen [ H] are deuterium, D or 2H & tritium, T or is 3H
He or helium is two on the Periodic Table of the atomic elements.
Carbon "formed in a star whenever three helium nuclei collide at one spot within less than a million millionth of a second. Every carbon atom in every living creature has been formed by such a wildly improbable collision."
Jacob Bronowski, The Ascent of Man.What binds the atomic parts – such as protons whose like charges repel one another – in ever more massive and divergent elements?
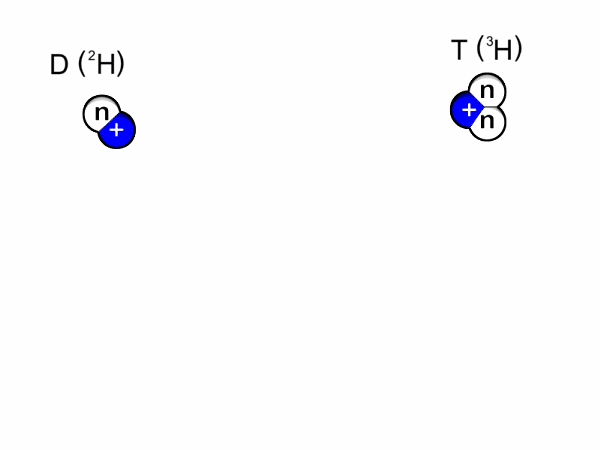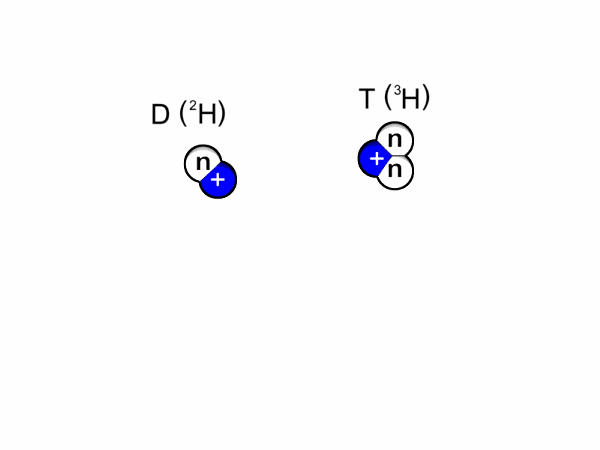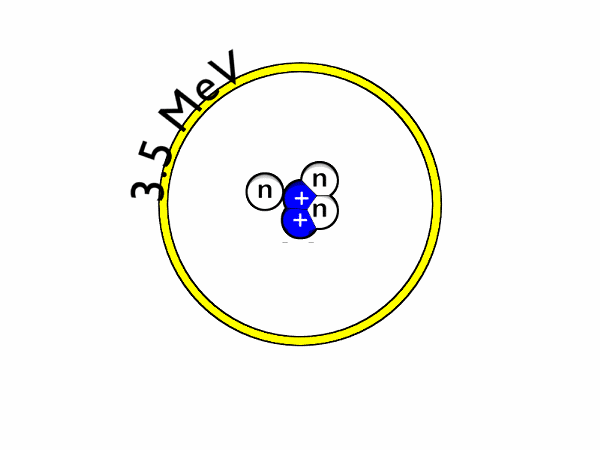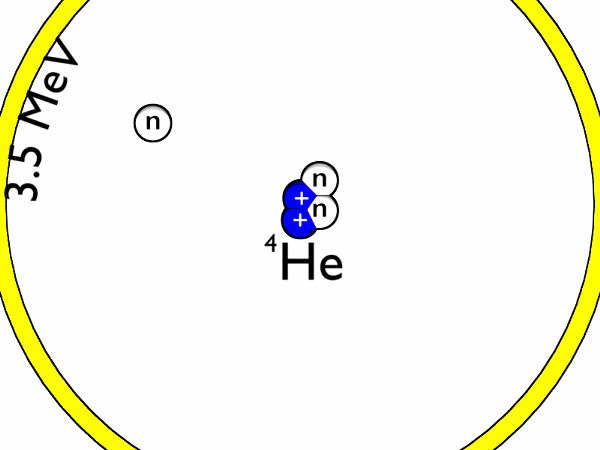
The binding energies of nucleons, or the particles within an atomic nuclei,are in the range of millions of electron volts compared to tens of eV for the force that holds atomic electrons around the nuclei. Whereas an atomic transition might emit a photon in the range of a few electron volts, perhaps in the visible light region, nuclear transitions in the atom's nucleus can emit gamma-rays with quantum energies in the MeV (millions of electron volts) range.
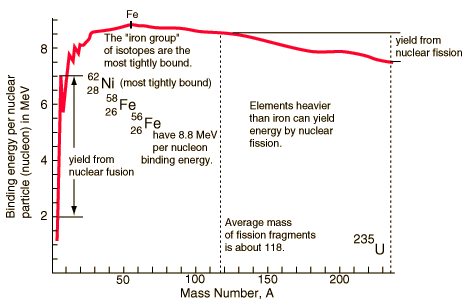
The curve is a quantitative and graphical depiction of the stability of protons and neutrons held by the strong force together and tightly packed in the nuclei of atoms.
The curve of binding energy graphically reveals that as the mass of the atoms increases and the ratio of neutrons to protons varies in heavier isotopes. There is less stability in those atomic elements whose mass is greater than iron than among those whose masses are less than iron [Fe]. The decline in stability at ever greater mass where protons and neutron ratios are closer to one to one means that certain more massive isotopes are susceptible to fission.


.gif)