"Can Nature possibly be as absurd as it seems to us in these atomic experiments?"
Werner Heisenberg, September, 1926.
Niels Bohr in 1913, described an atom mathematically, using the quantum theory of Max Planck. So counter-intuitive was the evidence that his model of the atom moved physics from the certainty of the macro-mechanical forces to the statistical variation in uncertainty of the quantum mechanical level of the very, very small micro cosmos.
"Yes, in what you say you are completely right. But that doesn't prove that there are no quantum jumps. It only proves that we can't visualise them, that means that the pictorial concepts we use to describe the events of everyday life and the experiments of the old physics do not suffice also to represent the process of a quantum jump. That is not surprising when one considers that the processes with which we are concerned here cannot be the subject of direct experience ... and our concepts do not apply to them."
Niels Bohr who worked with Ernest Rutherford.
For a model of the Bohr atom see this animation.
"Considering systems in which more electrons are bound by a positive nucleus, a configuration of the electrons which presents itself as a permanent state is one in which electrons are arranged in a ring around the nucleus."
Niels Bohr, February 1913, quoted by Ruth Moore, Niels Bohr: The Man, His Science, and the World They Changed. p.57.
A diagram of Bohr's concept of atoms
Bohr wrote:
"Following the theory of Rutherford, we shall assume that the atoms of the elements consist of a positively charged nucleus surrounded by a cluster of electrons. The nucleus is the seat of the essential part of the mass of atoms, and has linear dimensions exceedingly small compared with distances apart of the electrons in the surrounding cluster."
Ruth Moore, Niels Bohr. p.62.
The Bohr model of the hydrogen atom, (on the right) where negatively charged electrons confined to atomic shells encircle a small positively charged atomic nucleus, and that an electron jump (moving from one energy level to another) between orbits --that is the shells or clusters of electrons-- must be accompanied by an emitted or absorbed amount of electromagnetic energy hν.
As Bohr explained
". . . we shall assume that the cluster of electrons is formed by the successive binding by the nucleus of electrons initially nearly at rest, energy at the same time being radiated away."
Ruth Moore, Niels Bohr. p.63.
The orbits that the electrons travel in are shown as tan circles; their radius increases n2, where n is the principal quantum number. The 3→2 transition depicted here produces the first line of the Balmer series, and for hydrogen (Z = 1) results in a photon of wavelength 656 nanometers [nm] (red).
"Bohr assumed that a flash of energy would be radiated out as the captured electron was 'bound' into the nuclear orbit. Then he assumed that the energy given off in this action would equal one of Planck's quanta multiplied by the frequency of the rotation of the electron. This gave startling results, startling because they agreed with the figures obtained in experiments."
"Bohr's work was suggesting even more strongly . . . that the ring structure and its building up might not only make possible an understanding of the periodic system of the elements, but of how and why elements combine. It should be possible, Bohr added, to show why two hydrogen atoms will combine into a molecule 'while two helium-atoms won't.' "
Moore, Niels Bohr, p. 60.
The electron (red sphere negative sign) was discovered by J. J. Thomson in 1897.The nucleus of a Helium atom is called an alpha particle and was discovered by Rutherford in 1899.
Cambridge University physics outreach
UCSB Science line
see also Bohr atom model.
| field theory |
|---|
 |
The Bohr atom as he described in 1913 revolutionized our understanding of all atoms' material reality revealing a field of absorption and emission that came to be known as the spectral lines of an atom's bands of radiating frequencies.
"Energy is not emitted or absorbed in the continuous way physics assumed in the past, but only during the passing of systems between different 'stationary' states."
As Einstein is reported to have remarked upon hearing of the experimental confirmation of Bohr's model: "Then it is one of the great discoveries."
Moore, pp. 67-68.
| radiation levels | |||
| long wave | shorter wavelengths | ||
| low frequency | high frequency | ||
| red | violet | ||
 |
|||
base |
tenor |
soprano |
alto |
 |
|||
The nucleus; locus of the strong force, because this strong nuclear force overcomes the electromagnetic repulsive forces between protons inherent in atoms:
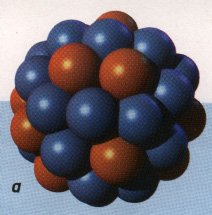 |
Atomic nuclei level |
|
|
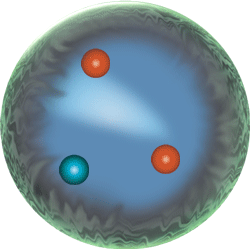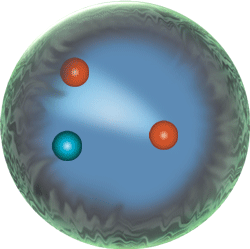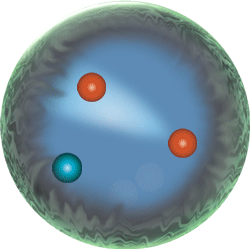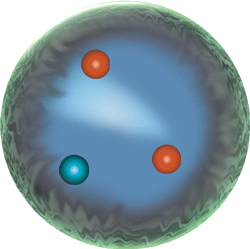 |
Sub-atomic particle level | neutrons, protons, electrons, mesons Because they are made of two up quarks and one down quark (uud),protons are baryons. So are neutrons because they are made of two down quarks and one up quark (udd). |
|
| "Quarks are elementary particles, building blocks of the atomic nucleus." ** | |||
ø |
+ |
charge |
Electrical energy as revealed in a magnetic field. |
terms |
The term "proton" first appeared in print by Ernest Rutherford, 1920. | ||
1932 |
after 1911 | discovered |
|
All atoms as we know today are composed of protons and some possess neutrons and electrons {a proton by itself is a Hydrogen ion 1H+} or a positively charged particle:
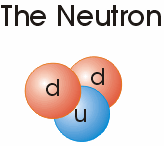 |
|
The proton & electron |
|---|---|---|
 |
||
| Atomic # | Mass # is equal to the total number of neutrons & protons | |
| Neutrons are 1842 times heavier than an electron | Protons are 1837 times heavier than an electron | Periodic table |
"Bohr explained that he was assuming that an atom is formed by the successive binding of electrons."
Moore, p. 51.
Nuclear and atomic properties compared not drawn to scale.
Sizes of the quantum world:
Planck scale:
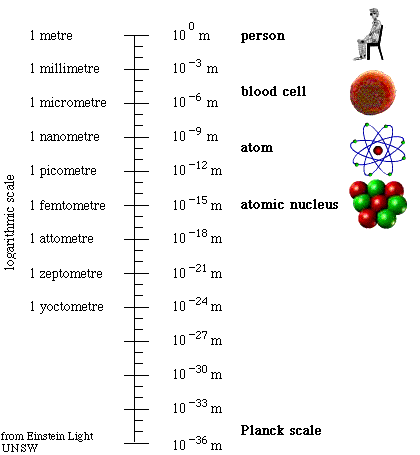
Diagram of the quantum levels of reality.
Layers revealing the quantum foam seething, deep down, beneath all of material reality the causes and effects we see in our world begin to melt away and do not have the anticipated outcomes of large-scale or celestial mechanics.
Level of organization organismic level atomic nucleus and electrons Bohr's atom in quantum reality
Electric charge is one physical property of matter that causes waves or particles to experience attractive or repulsive forces when placed in an electromagnetic field.
Quantum worlds of this femto universe
Artists' imagery of ever decreasing sizes at ever finer scales. Molecular level
∞
Atomic level
∞
Nucleus level
Quark level Spacetime is most definitely noisy.
The quantum fluctuations in spacetime were derived by John Wheeler in 1955. It is called quantum foam, spacetime foam, and Wheeler foam, in his honor [ the image at the bottom of these three drawings below ].
A nanometer is a measure of one billionth of a meter in extent.
Femto is from the Danish word for fifteen meaning 10-15.
atoms
Bohr's idea
complexity
doubt
Energy formula
photons
radiation as energy
See science animation web site from South Africa
Quantized energy states explained:
http://hyperphysics.phy-astr.gsu.edu/hbase/bohr.html
Certain considerations:
http://hyperphysics.phy-astr.gsu.edu/hbase/hyde.html#c2
sources: University of Oregon
http://abyss.uoregon.edu/~js/glossary/bohr_atom.html
Berkeley Lab's "The Particle Adventure"
* Ruth Moore, Niels Bohr: The Man, His Science, and the World They Changed. New York: Alfred Knopf, 1985.
** Murray Gell-Mann. The Quark and the Jaguar. p. 11.
Abraham Pais, Inward Bound: Of Matter and Forces in the Physical World.p. 208.