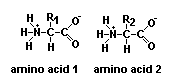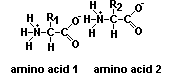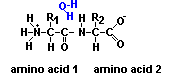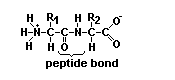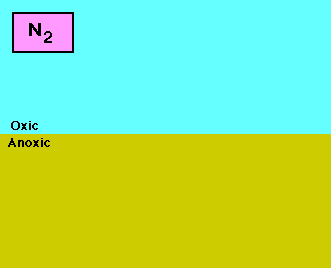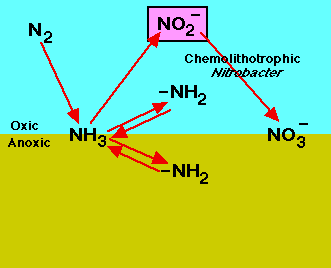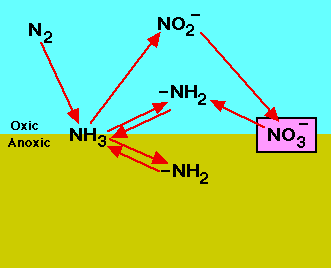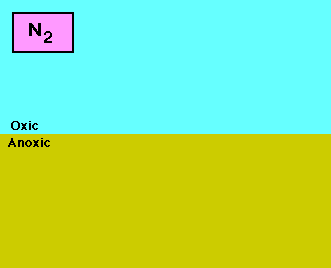|
 |
 |
Biological | Chemical | Geological
|
|
Biogeochemical
Cycles are ecological limiting factors

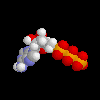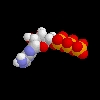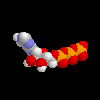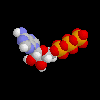 ATP, molecule that holds energy for life in the phosphate bonds ATP, molecule that holds energy for life in the phosphate bonds
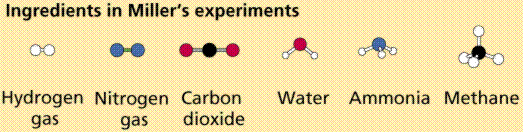
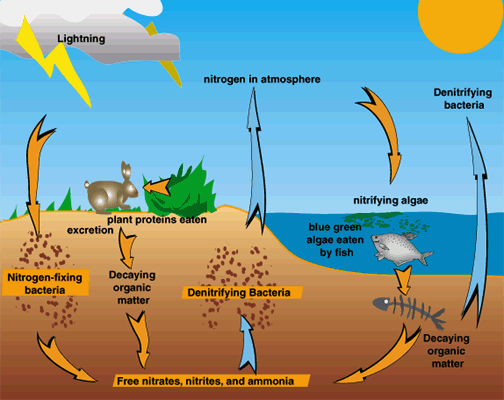
"some of the bacterial fermenters also developed the ability to absorb nitrogen gas from the air and convert it into various organic compounds. To 'fix' nitrogen–in other words to capture nitrogen directly form the air–takes large amounts of energy and is a feat that even today can be performed only by a few special bacteria. Since nitrogen is an ingredient of the proteins in all cells, all living organisms today depend on NITROGEN-FIXING BACTERIA for their survival."
Fritjof Capra, The Web of Life, pp. 236-237.
Water enables life.


|
Slow, variable,
& fast cycles.
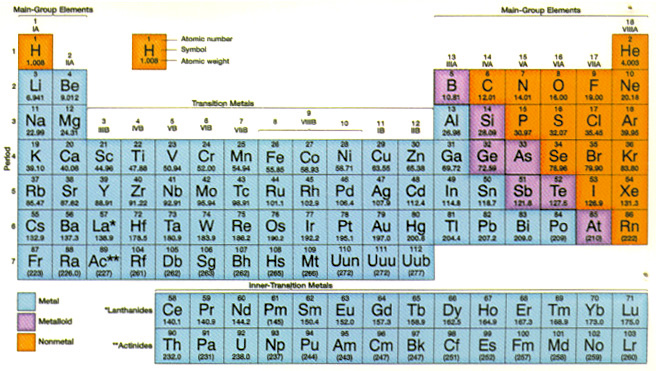
Periodic Table of the Atomic Elements
The roles of recurrent nutrient-cycles in the ecological dynamics sustaining life: nitrogen, sulfur, and carbon.
Return
to top of page
 |
The transport and removal or
deposition of vital atomic elements needed to
understand the dynamic qualities of ecological systems is embodies in a study of nutrient cycles.
Biogeochemical
cycles account for the characteristics, robustness and resilience of ecosystems
to withstand stress, rapid change, or population explosions. Justus Liebig
over 150 years ago defined a "law of the minimum," by which he understood
that the material element, or nutrient, needed in only trace amounts – but
necessary to the viability of production is the most serious
limiting factor. |
Carbon atoms arranged in a ball. |
| |
| |
| |
For
example, plants cannot photosynthesize without magnesium in
their chloroplasts. Magnesium is not needed in large amounts but is a significant
limiting factor because of its functional necessity. For plant-life magnesium is an excellent example of the law of the minimum. This is particularly
true in tropical climates where water and temperature conspire to increase the rate of evaporation and transpiration in plants.
Return
to top of page

Biogeochemical means that the biology of living creatures, the geological terrain and essential chemical elements are moved from the air, water, landscape and seas into and out of living parts of the world on a constant and recycling basis.
|

Return
to top of page

Biological means produced by living things.
So many living beings thrive together that symbiosis is an important factor in life.
Fuel, food, fiber and forage material are all produced by bacteria, fungi, plants or animals.
Virus and an associated bacteria.
Bacteria sustains all other forms of life.




Return
to top of page
 Chemical
refers to those compounds of an elemental character, referring to the trace matter, or nutrients that a living creature, or creatures sharing and ecosystem need to function properly.
Chemical
refers to those compounds of an elemental character, referring to the trace matter, or nutrients that a living creature, or creatures sharing and ecosystem need to function properly.
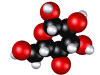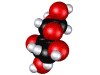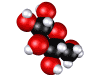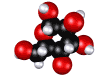 Glucose molecule; sugar is made by plants and is food for other forms of life.
Glucose molecule; sugar is made by plants and is food for other forms of life.
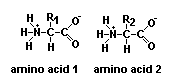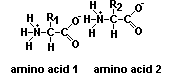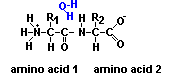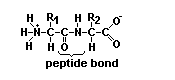 Proteins are essential for all forms of life and are made of chains of amino acids.
Proteins are essential for all forms of life and are made of chains of amino acids.
 Deoxyribonucleic acid or DNA – often shown as a double helix cartoon– is the heritable material of life.
Deoxyribonucleic acid or DNA – often shown as a double helix cartoon– is the heritable material of life.
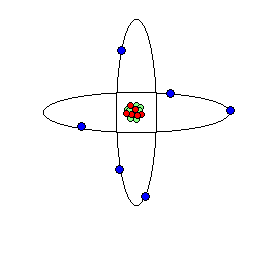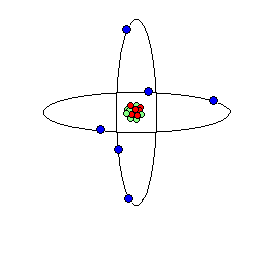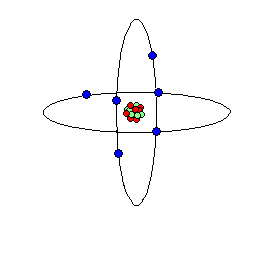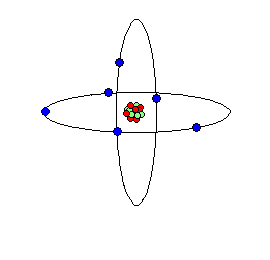 Carbon atom cartoon; carbon is the elemental basis of life on Earth.
Carbon atom cartoon; carbon is the elemental basis of life on Earth.

Return
to top of page
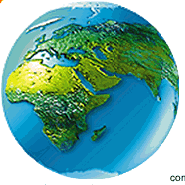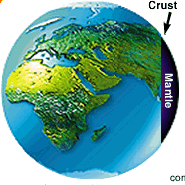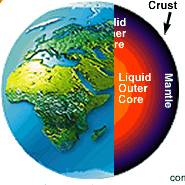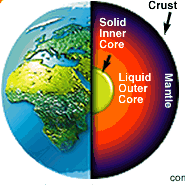 |
Geological |
|
| means both the time and the property
of belonging to the earth's processes even in the absence of living things,
elements predate life on earth. These elements are iron, silica,or magnesium
and compounds such as methane and ammonia. |
 |
 |
| |
|
 |
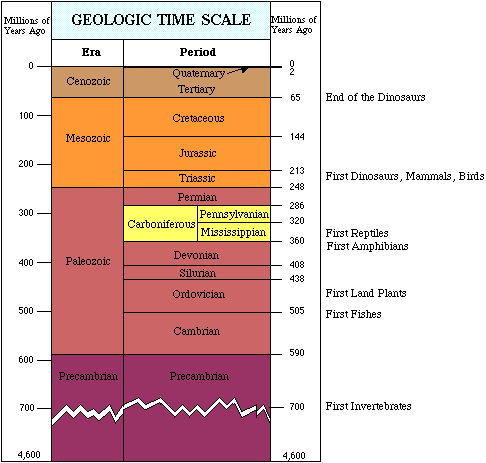 |
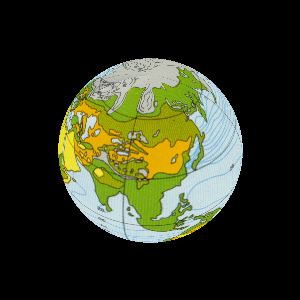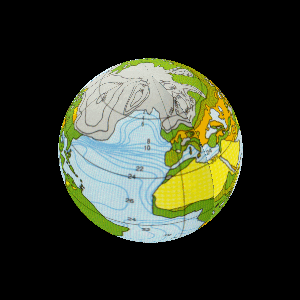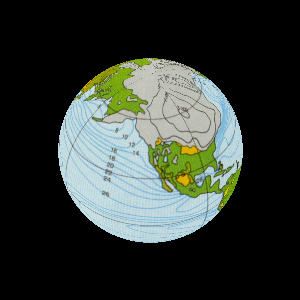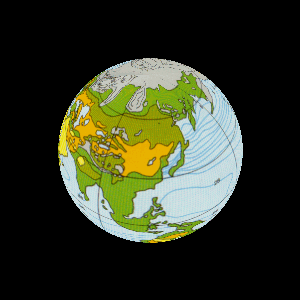
| The study of rock layers is called stratigraphy. |
For billions of years the Earth was home to bacteria. |
|
Return
to top of page
Different
conditions or states of matter on Earth:
gases
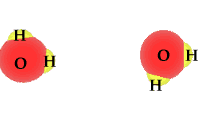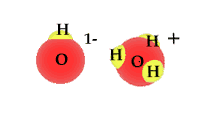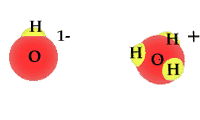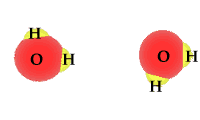
solids
liquids


H2O

"Water is the driver of life"
said Leonardo da Vinci.

Water is also the principle
mover, together with the
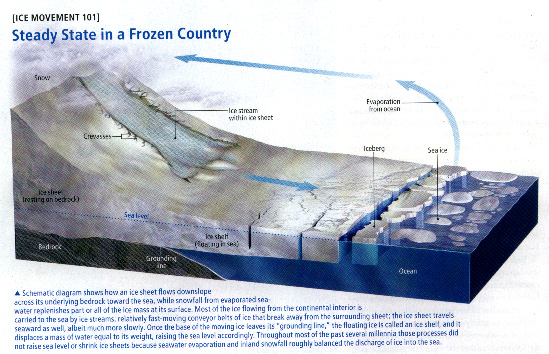
Earth's tectonic forces, of trace elements and nutrients through the air,
onto the land, into rivers, and oceans and into the rocks of the planet. As
the nutrients pass from one state and one place
to another they are made available for living organisms. This availability
is largely, but not always, the work of bacteria.

Return
to top of page
Places on Earth 
Air (atmospheric component) wind dispersal,
precipitation and temperature transport.
Water (marine and aqueous component) current
dispersal and removal by currents and evaporation.
Land (terrestrial component) evapotranspiration
through vegetation, animal and bacteria transport.
Underground (subsurface component) animal
and anaerobic bacteria transport.
Rocks (lithic component) tectonic (slow)
and biological (less slow) transport.
Return
to top of page

Slow, variable,
and fast cycles are moved by radiation
heating water and carrying these nutrients
throughout ecosystems.
The state of matter in
which nutrients are moved by inorganic forces has an important influence
on the availability of trace elements. Reservoirs or the total mass of these
compounds on earth are characterized by either readily soluble and thus
moving compounds as opposed to mostly insoluble and sluggish compounds.
Slow cycles can also occur due to the number of states of matter that the
compound element moves before it becomes available to bacteria.
Return
to top of page
gases are
most readily distributed from place to place.
Any atomic elements and compounds
of hydrogen, oxygen, nitrogen, sulfur and carbon all have a gas phase.
Return
to top of page
liquids are
readily moved by gravity from sources to sinks.
The hydrogen and oxygen compound
of water exists in each of these states on earth and the changes occur
in its condition or arrangement of molecules due to changes in temperature.
Normally a s gas at high (evaporation point: 162° F) temperatures,
water will freeze into a solid at 32° F, or will remain liquid in
the middle range of these temperatures, like other elements and compounds.
Return
to top of page
solids are
hard to transport from sinks where they accumulate.
Ice is a solid, but so are metals,
minerals and crystals, the heaviest and often most massive of compounds
and elements on earth. At high temperatures, such as in the core of the
Earth, iron, normally a solid exists as a liquid. The viscous magma from
volcanic explosions is an example of a super heated iron, nickel and mercury
compound that is, at surface temperatures, a solid.
Return
to top of page

C. Hopkins
Cafe 

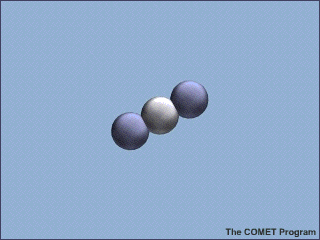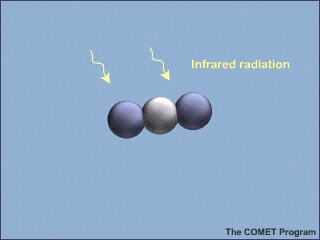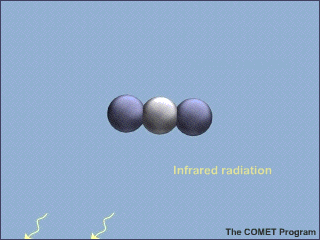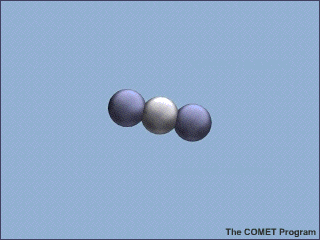
Carbon dioxide compound.
Nutrients
are those compounds or elements called trace elements, because they are so
scarce and they are needed only in the least amount, for necessary functions
of life in ecological systems to sustain itself and other forms of life.

"…Carbon, the chemical backbone of life, combined rapidly with hydrogen, oxygen, nitrogen, sulfur, and phosphorus to generate an enormous variety of chemical compounds. Those six elements–C, H, O, N, S, P–are now the main chemical ingredients in al living organisms."
Fritjof Capra, The Web of Life, p. 235.
qualities versus quantities
Return
to top of page
They come in two quantities based on how
much of the compound is needed for living things to function in a healthy
condition:
1. Major trace elements are: calcium, potassium,
magnesium, phosphorus, sulfur, carbon, oxygen, sodium, and nitrogen
2. Minor trace elements are: copper, cobalt,
manganese, molybdenum, boron, zinc, iodine, and iron.
Return
to top of page
Nutrients exist
with several qualities related to the frequency of their availability for living
creatures as they cycle quickly, slowly and with volatility.
Quickly
| Fast cycling and readily available: oxygen,
sodium and nitrogen, |
 |
 |
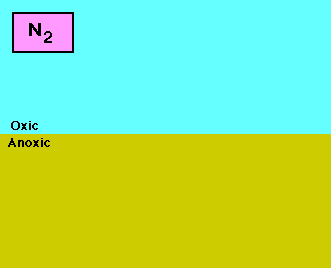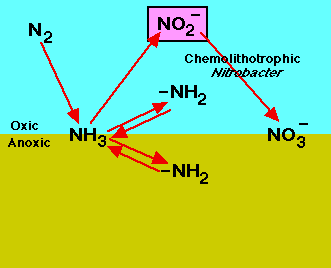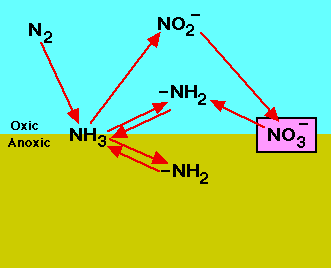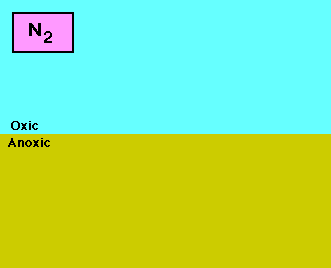 |
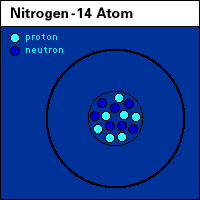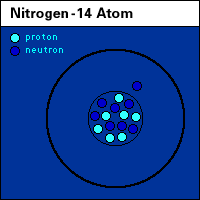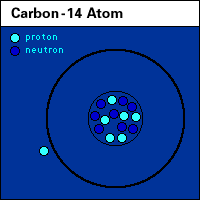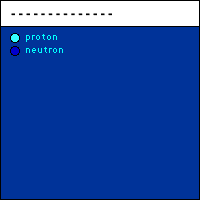
| Atomic element |
Roles of nitrogen in the land, air, and water. |
animated N, nitrogen cycle |
| |
|
|
Slowly
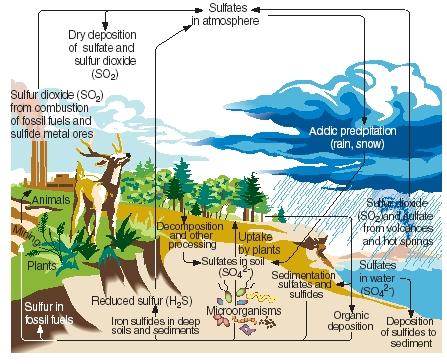
| Slow cycling and scarcely available: sulfur, calcium, potassium, magnesium, phosphorus, copper, cobalt, manganese,
molybdenum, boron, zinc, iodine, and iron. |
|
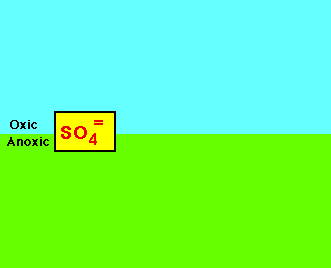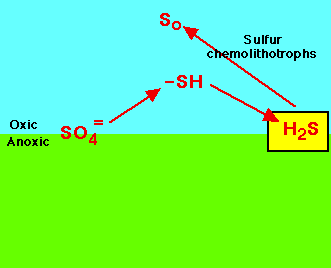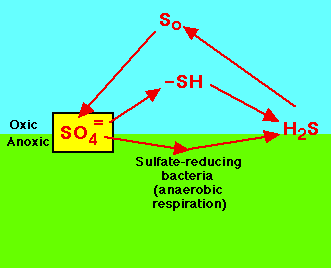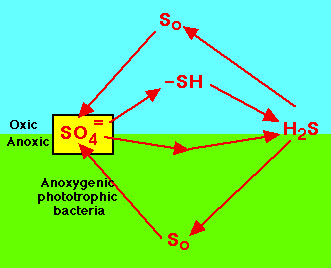 |
These are compounds often needed in the least amount and are examples of the law of the minimum. |
Return
to top of page
Irregular
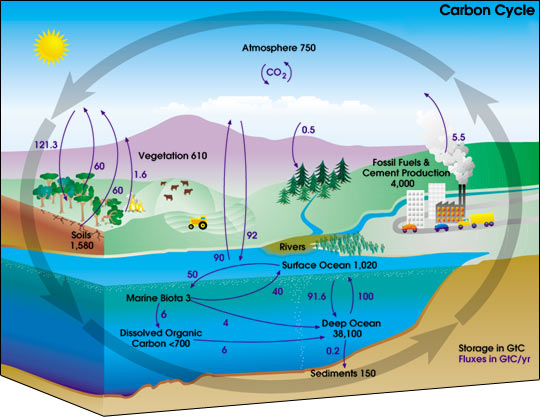
| Variable cycling: carbon. |
|
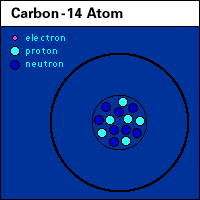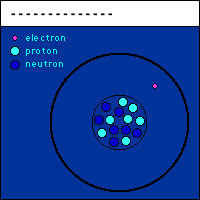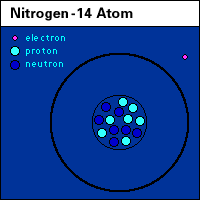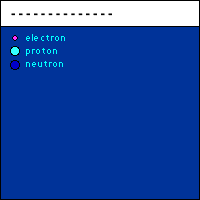 |
 |
 |
atomic structure |
compound molecule |
the biogeochemical cycle |
| Atoms |
Compounds |
Cycles |

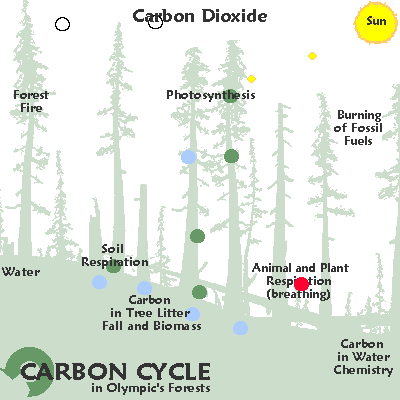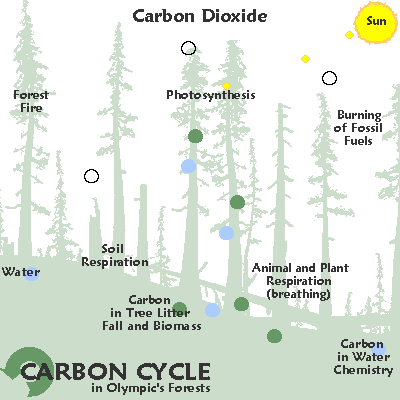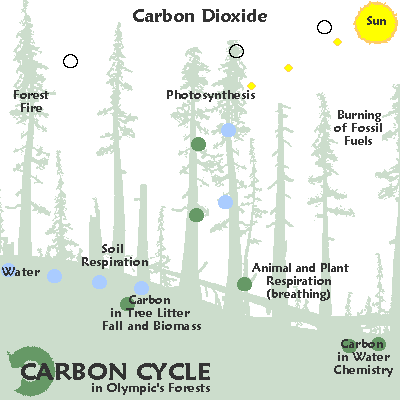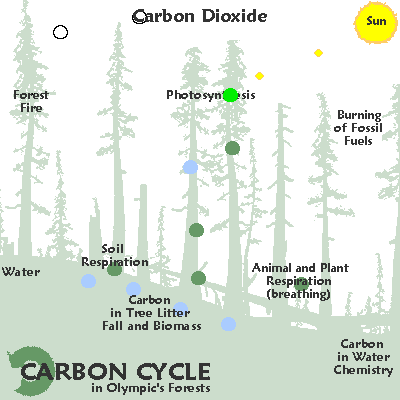
In the temperate rainforests of the Pacific rim carbon moves from the air into the ground an into cellulose due to photosynthesis.
A miraculous transformation, or as Oliver Sacks has suggested:
"It is impossible to imagine a world without photosynthesis . . . this most important life process."

The Earth's Green Mantle
More
on life sciences
More on nutrients
Return
to top of page
Words we use | Meaning | analysis | a sample definition | ecology | ecological terms | model | habitat

Science
search this site
contents
of this web site
Darwin | Mayr | Ehrlich | Faulkner | Hardin | Hooke | Bronowski | Tattersall | Margulis | Miller | Wilson | Einstein's equation | Thomas


















 Chemical
refers to those compounds of an elemental character, referring to the trace matter, or nutrients that a living creature, or creatures sharing and ecosystem need to function properly.
Chemical
refers to those compounds of an elemental character, referring to the trace matter, or nutrients that a living creature, or creatures sharing and ecosystem need to function properly.  Glucose molecule; sugar is made by plants and is food for other forms of life.
Glucose molecule; sugar is made by plants and is food for other forms of life.