 fast and slow elemental cycles
fast and slow elemental cycles
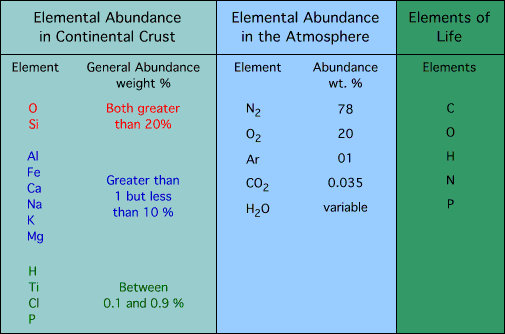
The above phrase –Chopkins cafe– is an acronym used by folks to easily recall the ten vital atomic elements needed to understand life on Earth based on the dynamic, essential, and inherent qualities of biogeochemical cycles.
laws of ecology | laws of thermodynamics | universal laws
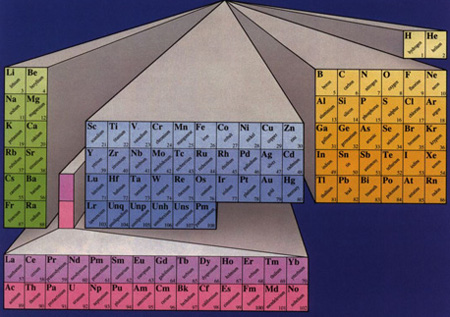
Atomic means pertaining to the 110 or so elements on the periodic table arranged according to their masses and their requisite numbers of protons and neutrons.
Elements refers to any basic building block that accounts for the function or the underlying cause of things.
Dynamic refers to changes that occur over time and the cycles refer to the recurrent patterns in these alterations of material from one condition to another in the course of a measurable duration.
C H O P K I N S C A F E
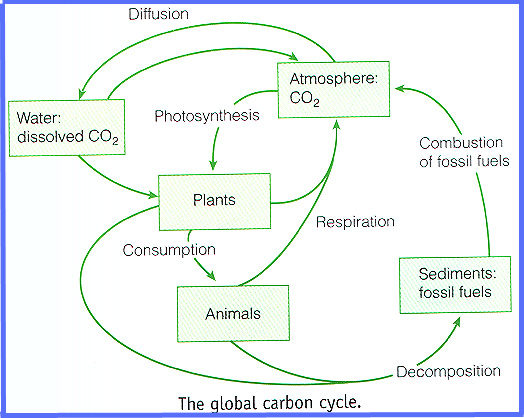
Types of materials cycled on the earth:
Compounds:
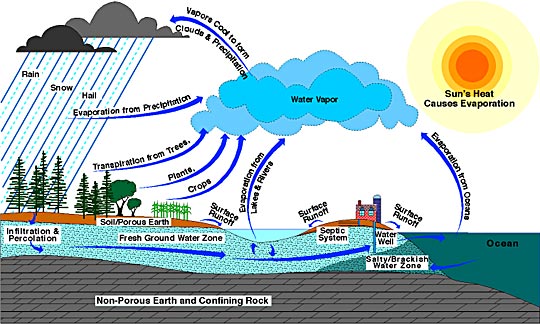
Slow and fast cycles are moved by radiation heating water and carrying these nutrients throughout ecosystems.
H for hydrogen: a most abundant, gas
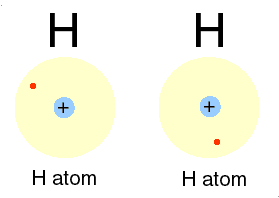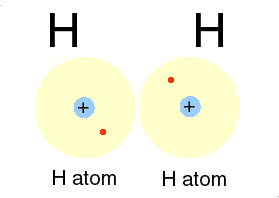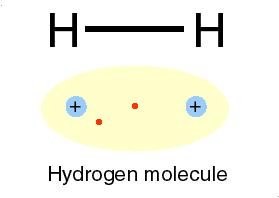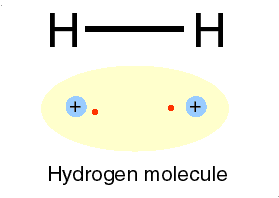
![]() O for oxygen: faster cycling, gas
O for oxygen: faster cycling, gas
 N for nitrogen: fast cycling
, gas
N for nitrogen: fast cycling
, gas
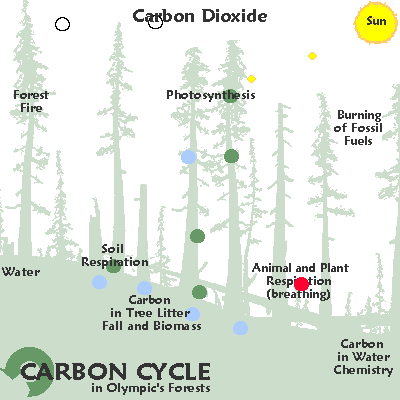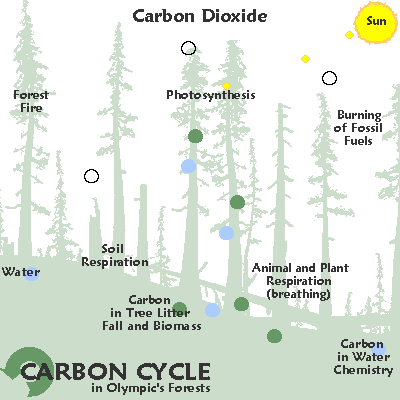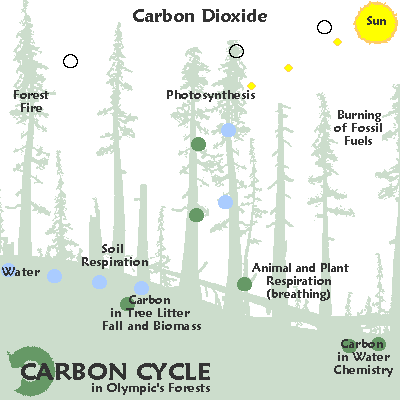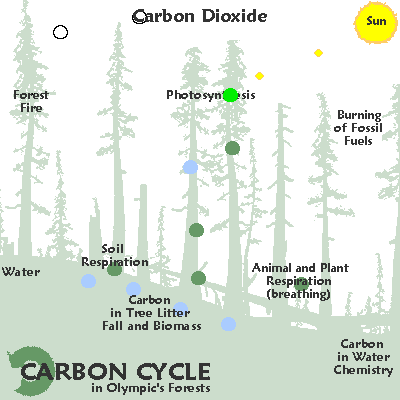
C for carbon: variable as fast or slow cycling, gas / solid / liquid
phosphorus – sulfur – potassium – calcium – carbon
P for phosphorus: slow cycling, solid
• used by living things for energy metabolism: ATP.
S for sulfur: slow cycling, solid
• sulfur bonds are used by living things shaping the structure of functioning enzymes.
K for potassium: slower cycling, solid
• used by living things for regulating enzymatic responses in plants & electrolytes in animals.
Ca for calcium: slowest cycling, solid
• used by living things in cellular microtubules and shells, bones, or teeth.
 C for carbon: variable cycling , solid
/ liquid / gas
C for carbon: variable cycling , solid
/ liquid / gas
Limestone –pictured here–is a compound of calcium and carbon.
Mg for magnesium slowest cycling, Mineral
Me for mercury variable cycling, Mineral
Nutrients come in two quantities:
1. Major trace elements are: calcium, potassium, magnesium, phosphorus, sulfur, carbon, oxygen, sodium and nitrogen
2. Minor trace elements are: copper, cobalt, manganese, molybdenum, boron, zinc, iodine, and iron.
Nutrients come in two qualities:
Fast cycling and readily available; oxygen, sodium and nitrogen, sulfur,
Slow cycling and scarcely available; calcium, potassium, magnesium, phosphorus, copper, cobalt, manganese, molybdenum, boron, zinc, iodine, and iron.
Variable rate of cycling: carbon.
Biogeochemical cycles account for the characteristics, robustness and resilience of ecosystems to withstand stress, rapid change, or population explosions. Justus Liebig over 150 years ago defined a "law of the Minimum," by which he understood that the material element, or nutrient, needed in only trace amounts -- but necessary to the viability of production is the most serious limiting factor. Limiting factors such as nutrient deficiencies can undermine healthy and hence productive crops, organisms and biotic communities.
For example, plants cannot photosynthesize without magnesium in the chloroplasts. Magnesium is not needed in large amounts but is a serious limiting factor and an example of the law of the minimum. This is particularly true in tropical climates.
Periodic Table of the Atomic Elements
Quantum universe